A new method developed by NIST uses infrared light to image biomolecules in cells, overcoming previous limitations caused by water absorption. This technique aids in the analysis of proteins and other biomolecules, facilitating progress in biotechnology and medicine.
IST’s new infrared microscopy technique allows for the detailed imaging of biomolecules in cells, supporting advancements in biotechnology and cellular therapies.
In an effort to advance biotechnology innovations, scientists are working to develop faster, more quantitative, and more accessible ways to observe biomolecules in living cells.
Now, researchers at the National Institute of Standards and Technology (NIST) have developed a new method that allows the use of infrared (IR) light to capture clear images of biomolecules inside cells, something that was previously not possible due to the tendency of the water in cells to absorb infrared radiation.
NIST’s new method removes the obscuring effects of water in IR-based measurements and allows researchers to determine the amounts of key biomolecules in cells, such as the proteins that direct cell function. The ability to measure changes in living cells could speed up advances in biomanufacturing, cell therapy development, drug development, and more.
Their findings have been published in Analytical Chemistry.
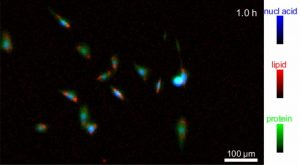
An image of biomolecules, such as nucleic acids, lipids and proteins, in live cells using an imaging technique called infrared (IR) transmission microscopy. Credit: Y. Lee/NIST
Understanding Infrared Microscopy and Its Challenges
Infrared radiation is light that is just beyond what is visible to the human eye. Although we cannot see IR light, we can feel it as heat. In IR microscopy, a material of interest absorbs radiation from a range of wavelengths in the IR spectrum. Scientists measure and analyze the IR absorption spectrum of a sample, producing a set of “fingerprints” to identify molecules and other chemical structures. However, water, the most abundant molecule both inside and outside cells, absorbs infrared strongly and masks the infrared absorption from other biomolecules in cells.
One way to understand this optical masking effect is to compare it to when an airplane passes overhead next to the Sun. With the naked eye, it’s hard to see the airplane because of the Sun, but if you use a special Sun-blocking filter, then you can easily see the airplane in the sky.
“In the spectrum, water absorbs infrared so strongly, and we want to see the absorption spectrum of proteins through the thick water background, so we designed the optical system to uncloak the water contribution and reveal the protein signals,” said NIST chemist Young Jong Lee.
Advancing Cellular Analysis with SAC-IR
Lee developed a patented technique that uses an optical element to compensate for water absorption from IR. Called solvent absorption compensation (SAC), the technique was used with a hand-built IR laser microscope to image cells that support the formation of connective tissue, called fibroblast cells. Over a 12-hour observation period, researchers were able to identify groups of biomolecules (proteins, lipids, and nucleic acids) during stages of the cell cycle, such as cell division. While this may seem like a long time, the method is ultimately faster than current alternatives, which require beam time at a large synchrotron facility.
This new method, called SAC-IR, is label-free, meaning it does not require any dyes or fluorescent markers, which can harm cells and also produce less consistent results across labs.
The SAC-IR method enabled NIST researchers to measure the absolute mass of proteins in a cell, in addition to nucleic acids, lipids, and carbohydrates. The technique could help establish a foundation for standardizing methods for measuring biomolecules in cells, which could prove useful in biology, medicine, and biotechnology.
“In cancer cell therapy, for example, when cells from a patient’s immune system are modified to better recognize and kill cancer cells before being reintroduced back to the patient, one must ask, ‘Are these cells safe and effective?’ Our method can be helpful by providing additional insight with respect to biomolecular changes in the cells to assess cell health,” said Lee.
Other potential applications include using cells for drug screening, either in the discovery of new drugs or in understanding the safety and efficacy of a drug candidate. For example, this method could help to assess the potency of new drugs by measuring absolute concentrations of various biomolecules in a large number of individual cells or to analyze how different types of cells react to the drugs.
Future Applications and Improvements
The researchers hope to develop the technique further so it can measure other key biomolecules, such as DNA and RNA, with greater accuracy. The technique could also help provide detailed answers to fundamental questions in cell biology, such as what biomolecule signatures correspond with cell viability — in other words if the cell is alive, dying, or dead.
“Some cells are preserved in a frozen state for months or years, then thawed for later use. We don’t yet fully understand how best to thaw the cells while maintaining maximum viability. With our new measurement capabilities, we may be able to develop better processes for cell freezing and thawing by looking at their infrared spectra,” said Lee.
Reference: “Benchtop IR Imaging of Live Cells: Monitoring the Total Mass of Biomolecules in Single Cells” by Yow-Ren Chang, Seong-Min Kim and Young Jong Lee, 4 September 2024, Analytical Chemistry.
DOI: 10.1021/acs.analchem.4c02108
SciTechDaily – September 19, 2024
By National Institute of Standards and Technology (NIST)